TE-BC251 NIOSH Bioaerosol Cyclone
Developed by the National Institute for Occupational Safety and Health (NIOSH) and manufactured by Tisch Environmental, the TE-BC251 is a wearable multi-stage bio-aerosol collector used for the collection of airborne viruses including INFLUENZA and COVID-19. This high quality, low cost cyclone is well suited for quick deployment to respond to the current rapidly evolving climate. The multi stage cyclone is designed to trap bio-aerosols in one of three compartments for accurate retrieval of samples at multiple cut points.
At 3.5 l/min, the BC 251 conforms to the ACGIH/ISO criterion for separation of respirable and non-respirable airborne particles, which is widely used in health-related aerosol measurements. The BC 251 has been successfully used to collect airborne influenza virus in healthcare facilities and from the coughs of influenza patients. The sampler can be operated with commercially-available personal air sampling pumps. This cyclone is now available with a backpack to hold both the cyclone and associated pump during testing. A tripod stand is also available for free standing testing.
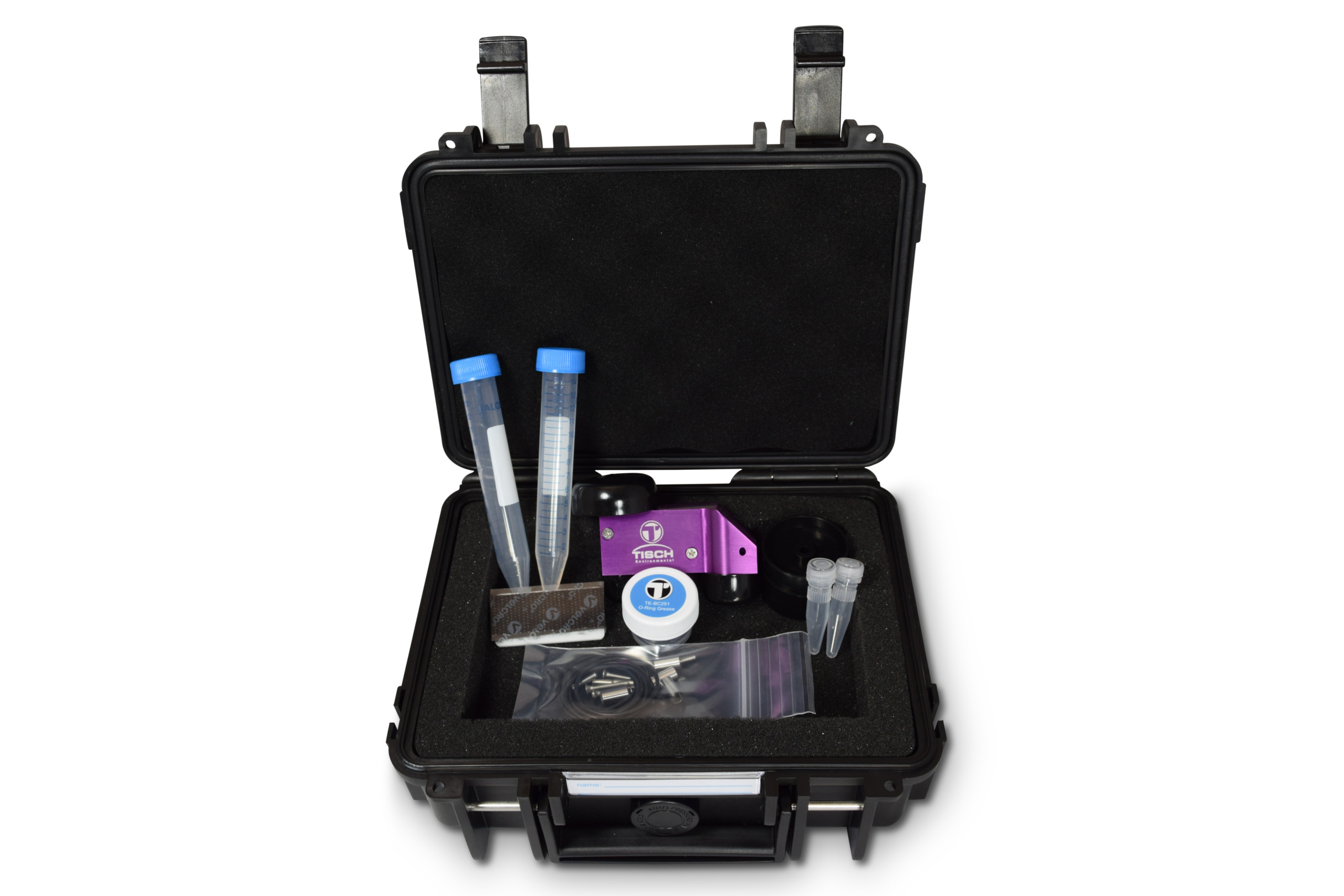
Each sampler comes with the following:
- (1) BC251 Cyclone
- (1) Heavy Duty Carrying Case
- (2) Falcon™ 15 mL Conical Centrifuge Tubes
- (2) Fisherbrand™ Nonsterile Threaded End Microcentrifuge Tube without Caps
- (2) Fisherbrand™ Colored Screw Caps
- (1) Jar of O-ring grease
- (10) TE-BC251-5 EPDM O-Ring, 1/16 Width Dash Number 027
- (1) Filter Cassette
| flow rate (lpm) |
1st stage 50% cut-off size (um) |
1st stage sharpness (geometric standard deviation) |
2nd stage 50% cut-off size (um) |
2nd stage sharpness (geometric standard deviation) |
|---|---|---|---|---|
| 2 | 4.9 | 1.48 | 1.7 | 1.68 |
| 3.5 | 4.1 | 1.51 | 1.0 | 1.59 |
| 10 | 2.1 | 1.44 | 0.41 | 1.56 |
Notes on using the BC samplers
NIOSH BC 251 cyclone aerosol sampler publications 2020-05-18
Fennelly, KP (2020). Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med 8(9): 914-924. https://www.ncbi.nlm.nih.gov/pubmed/32717211
Gralton, J, ER Tovey, ML McLaws and WD Rawlinson (2013). Respiratory virus RNA is detectable in airborne and droplet particles. J Med Virol 85(12): 2151-9. https://www.ncbi.nlm.nih.gov/pubmed/23959825
Leung, NHL, DKW Chu, EYC Shiu, KH Chan, JJ McDevitt, BJP Hau, HL Yen, Y Li, DKM Ip, JSM Peiris, WH Seto, GM Leung, DK Milton and BJ Cowling (2020). Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 26(5): 676-680. https://www.ncbi.nlm.nih.gov/pubmed/32371934
Lindsley, WG, FM Blachere, DH Beezhold, RE Thewlis, B Noorbakhsh, S Othumpangat, WT Goldsmith, CM McMillen, ME Andrew, CN Burrell and JD Noti (2016). Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir Viruses 10(5): 404-13. https://www.ncbi.nlm.nih.gov/pubmed/26991074
Lindsley, WG, TA Pearce, JB Hudnall, KA Davis, SM Davis, MA Fisher, R Khakoo, JE Palmer, KE Clark, I Celik, CC Coffey, FM Blachere and DH Beezhold (2012). Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg 9(7): 443-9. https://www.ncbi.nlm.nih.gov/pubmed/22651099
Milton, DK, MP Fabian, BJ Cowling, ML Grantham and JJ McDevitt (2013). Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 9(3): e1003205. https://www.ncbi.nlm.nih.gov/pubmed/23505369
Pan, M, JA Lednicky and CY Wu (2019). Collection, particle sizing and detection of airborne viruses. J Appl Microbiol 127(6): 1596-1611. https://www.ncbi.nlm.nih.gov/pubmed/30974505
Yan, J, M Grantham, J Pantelic, PJ Bueno de Mesquita, B Albert, F Liu, S Ehrman, DK Milton and E Consortium (2018). Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA 115(5): 1081-1086. https://www.ncbi.nlm.nih.gov/pubmed/29348203
Binder, RA, NA Alarja, ER Robie, KE Kochek, L Xiu, L Rocha-Melogno, A Abdelgadir, SV Goli, AS Farrell, KK Coleman, AL Turner, CC Lautredou, JA Lednicky, MJ Lee, CR Polage, RA Simmons, MA Deshusses, BD Anderson and GC Gray (2020). Environmental and Aerosolized Severe Acute Respiratory Syndrome Coronavirus 2 Among Hospitalized Coronavirus Disease 2019 Patients. J Infect Dis 222(11): 1798-1806. https://www.ncbi.nlm.nih.gov/pubmed/32905595
Birgand, G, N Peiffer-Smadja, S Fournier, S Kerneis, FX Lescure and JC Lucet (2020). Assessment of Air Contamination by SARS-CoV-2 in Hospital Settings. JAMA Netw Open 3(12): e2033232. https://www.ncbi.nlm.nih.gov/pubmed/33355679
Dabisch, P, M Schuit, A Herzog, K Beck, S Wood, M Krause, D Miller, W Weaver, D Freeburger, I Hooper, B Green, G Williams, B Holland, J Bohannon, V Wahl, J Yolitz, M Hevey and S Ratnesar-Shumate (2020). The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci Technol 55(2): 142-153. https://doi.org/10.1080/02786826.2020.1829536
Edwards, DA, D Ausiello, J Salzman, T Devlin, R Langer, BJ Beddingfield, AC Fears, LA Doyle-Meyers, RK Redmann, SZ Killeen, NJ Maness and CJ Roy (2021). Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc Natl Acad Sci USA 118(8): e2021830118. https://www.ncbi.nlm.nih.gov/pubmed/33563754
Lane, MA, EA Brownsword, A Babiker, JM Ingersoll, J Waggoner, M Ayers, M Klopman, TM Uyeki, WG Lindsley and CS Kraft (2021). Bioaerosol sampling for SARS-CoV-2 in a referral center with critically ill COVID-19 patients March-May 2020. Clin Infect Dis. https://www.ncbi.nlm.nih.gov/pubmed/33506256
Lednicky, JA, M Lauzardo, ZH Fan, A Jutla, TB Tilly, M Gangwar, M Usmani, SN Shankar, K Mohamed, A Eiguren-Fernandez, CJ Stephenson, MM Alam, MA Elbadry, JC Loeb, K Subramaniam, TB Waltzek, K Cherabuddi, JG Morris, Jr. and CY Wu (2020). Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 100: 476-482. https://www.ncbi.nlm.nih.gov/pubmed/32949774
Lednicky, JA, SN Shankar, MA Elbadry, JC Gibson, MM Alam, CJ Stephenson, A Eiguren-Fernandez, JG Morris, CN Mavian, M Salemi, JR Clugston and C-Y Wu (2020). Collection of SARS-CoV-2 Virus from the Air of a Clinic within a University Student Health Care Center and Analyses of the Viral Genomic Sequence. Aerosol Air Qual Res 20(6): 1167-1171. https://dx.doi.org/10.4209/aaqr.2020.02.0202
Lei, H, F Ye, X Liu, Z Huang, S Ling, Z Jiang, J Cheng, X Huang, Q Wu, S Wu, Y Xie, C Xiao, D Ye, Z Yang, Y Li, NHL Leung, BJ Cowling, J He, SS Wong and M Zanin (2020). SARS-CoV-2 environmental contamination associated with persistently infected COVID-19 patients. Influenza Other Respir Viruses 14(6): 688-699. https://www.ncbi.nlm.nih.gov/pubmed/32578948
Ong, SWX, YK Tan, KK Coleman, BH Tan, YS Leo, DL Wang, CG Ng, OT Ng, MSY Wong and K Marimuthu (2021). Lack of viable severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among PCR-positive air samples from hospital rooms and community isolation facilities. Infect Control Hosp Epidemiol: 1-6. https://www.ncbi.nlm.nih.gov/pubmed/33487210










Get Social